water and vinegar
Water of course is the most important solvent on our planet, and the varieties and qualities of water vary greatly from city water, to spring water, to rain and dew. Extractions from water alone will spoil much more easily than from alcoholic tinctures, especially without refrigeration. We will not discuss herbal teas and infusions as common herbal literature contains much information.
Another solvent that deserves brief mention is vinegar. Vinegar is also a vegetable fermentation product. Its extractive power comes from its acidity and has been used in herbal medicines since ancient times. Plants are macerated in vinegar for a few weeks, then the vinegar is used as a basic tincture. Natural vinegar can be used directly or concentrated by freezing. Apple cider vinegar, grape and wine vinegars are considered the best. The traditional candidates for vinegar tinctures are the Labiatae family (mint, oregano, basil, etc.).
The main alternatives to ethyl alcohol we will discuss are as follows:
olive oil
Olive oil is not an essential oil (the olive flower does produce an oil used in aromatherapy, but that is a different oil) but is the fat of the fruit itself. While many fats and oils can be used for extraction of essentials of plants and herbs, olive oil is particularly a powerful solvent and it has been used in Mediterranean homes in hundreds of applications from skin care to cleaning stains.

Some may question the use of an oil as a Mercury in the plant kingdom; however, the plant kingdom itself is very complicated and composited and it is almost impossible to find a pure philosophical product. Even alcohol itself, no matter how many times distilled or dehydrated, will still carry a trace of the original substance from which it was fermented. It is called, in some circles, a "Sulphuric Mercury" indicating its contamination with a sulphur from its own origin. For these reasons we feel justified in using olive oil solvent as a plant kingdom "Sulphuric Mercury" as it performs a similar function.
The only olive oil we recommend to use is "extra virgin" olive oil produced by mechanical means. Pomace oil varieties are produced by solvent extraction and thus are least desirable. The best virgin oil is that which is less than a year old and which typically has a mild bitter taste; however, store bought virgin oil works well also.

Swedish Bitters
Hot maceration
The primary application of olive oil we will consider here is the making of spagyric tinctures by maceration. Olive oil works well for seeds and dry plants with fat soluble contents such as echinacea, arnica, chamomile and even edible mushrooms. The powers of these substances can be extracted by warm maceration (40-50°C) in extra virgin olive oil. Let the maceration continue for a week or two, then remove the plant residues by filtration. The tinged oil can be used directly or we can add the water soluble plant salts and circulate for another week. Fresh plants can be used, of course, but it is best to dry them as any moisture in the olive oil can produce rancidity over time. Of course some compounds are not fat soluble. An example would be flowering ash manna in Swedish Bitters. Almost all of the herbs in the Swedish Bitters mix work well with olive oil, except manna which is water soluble. In this case, the Bitters mix can be formulated with other alternatives such as Melissa or any other herb or resin specific to the liver.
limonene or orange oil
Another powerful "Sulphuric Mercury" of the plant kingdom is limonene. Orange oil is about 90% limonene. Limonene is not an oil but actually a terpenes, a natural hydrocarbon. It is extracted from the peel of citrus fruit such as oranges and lemons. This hydrocarbon solvent is food safe, biodegradable, completely nontoxic to humans, widely available and is itself classed as a natural medicine. It is known to have anti-stress properties and is also a mild sedative.

While being safe for humans, it is a very powerful non-polar solvent; more powerful than naphtha or mineral spirits. It boils at 175°C. It is fully soluble (miscible) in alcohol and its industrial applications are numerous from cleaning concrete and industrial degreasing to use as an eco-friendly pesticide. In citrus producing countries, food grade orange oil can be obtained by the liters or gallons.
When buying limonene, ensure that it is food grade. Limonene products in hardware stores for wood treatment contain other additives and adulterants that are usually toxic. Alternatively, you can obtain your own limonene by steam distillation of orange peel; but ensure that you have organic citrus as citrus fruits are usually heavily sprayed with pesticides. Cold pressing the peel is another way to extract limonene if you have access to a strong vegetable press. The orange oil can be further distilled to separate the limonene mercurial spirit from the oily remains of the citrus rind.
Applications of limonene can cover a wide range of alchemical processes; however, it is most useful in the Ens process in particular.
limonene in the Ens process
This is where limonene shines. The fresh plant is crushed and made into a paste-like consistency. Deliquescent potash (or any strong food safe alkali) is added and left for a day or two. Modern alchemical lore holds that the rectified spirit of wine is added to the mix, shaken and then left to separate into layers. The alcohol layer contains the Ens.
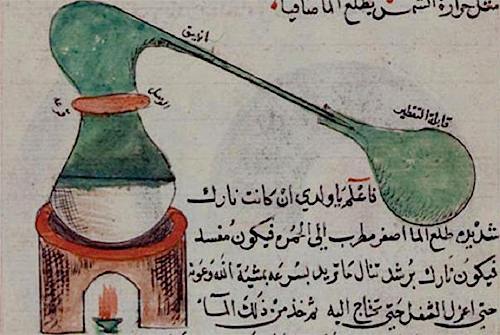
However, with our modern knowledge of chemistry we know that alcohol is not a good solvent to extract freebase medicines in the alkali/plant mix. Alcohol is polar and a non-polar solvent is needed in this case. This is one reason we may suspect the spirit of wine of the Ens process is not ethyl alcohol but rather a non-polar solvent of which only one was mentioned in ancient alchemical literature, Diethyl Ether. It was then called the Sweetened Spirit of Vitriol first described by Jabir Ibn Hayyan [The evolution of anaesthesia, Br. J. Anaesth. (1958) 30 (10): 495-5040] and it is obtained by sharpening (or sweetening as Basil Valentine calls it) oil of vitriol with rectified alcohol. The legendary alchemist and knight, Raymond Lully, also described the use of this special solvent. The oil of vitriol was the red essence obtained by dry distillation of vitriols. When mixed with rectified alcohol and distilled, it yields ether which needs to be condensed at temperatures less than 34°C.

With limonene we can avoid the complexity of the process and use Limonene as a substitute for the Spirit of Wine (alcohol or sweetened spirit of vitriol). Like ether, limonene is a non-polar solvent and will readily extract all the non-water soluble compounds and freebase medicines in the plant/potash mix. Recall that the Ens process relies on highly rectified spirits which indicates that it is targeting non-water soluble substances The limonene layer will contain the Ens. Limonene can be consumed directly as it is more difficult to evaporate than ether or alcohol. If consumed directly, the strongly tinged limonene should be consumed in less than a gram per day, which is about 15 drops per day as a maximum, but for the Ens process 5 drops is the customary daily dose.
glycerol or glycerine
Glycerol is found in all cellular life forms in the form of triglyceride formed from photosynthesis produced carbohydrates and plays a significant role in the metabolism of living organisms. Triglycerides are fatty acids bound to glycerol and constitute the basis of almost all animal and vegetable fats and oils. Additionally, it has the combined solvent characteristics of both alcohol and water. Thus we consider that glycerol is a vegetable Mercury par excellence. It can also be produced by fermentation of plant sugars in a marvellous canonical process similar to alcohol production by fermentation, with the resulting product being a mix of alcohol/glycerol. We discuss this process in a separate paper which is circulated within Inner Garden.

Glycerol is also produced as a by-product of soap making, another ancient process known to the Egyptians and Akkadians. Biodiesel production also yields glycerol as a by-product and it is the main source of "vegetable" glycerol. Non vegetable glycerol is produced from petroleum products and is of no interest to us due to possible toxic contamination. Glycerol has numerous applications in the pharmaceutical and the cosmetic industries. Glycerol is soluble in both water and alcohol but not oils. It is hygroscopic and will absorb air moisture until it has a 20% water content. It is difficult to distil due to its high boiling temperature (290°C) and the fact that it decomposes before reaching boiling temperature under normal conditions, thus it can be practically distilled only under vacuum (10mbar at 160°C).
Glycerol's extractive power is similar to alcohol in that it is a polar solvent but a little weaker and hence requires longer maceration times (1.5-2 times longer) if used for basic tincture extractions.
The only glycerol we should buy from stores is vegetable glycerol of food grade. You can buy it by the gallon in some stores and in most pharmacies in small quantities, which is best, as we can use it when needed to keep it from absorbing atmospheric moisture (treat it as rectified and desiccated alcohol). Glycerol can also be made through a home biodiesel process but this involves the use of Methanol that will end up in the glycerol water mix and thus requires specialized treatment to be safe. Another process is by saponification of oils. It is simple to make and safe but you end up with a glycerol/salt/water mix which needs to be vacuum distilled to separate the glycerol from the salt.
Glycerol can be used as a 1-1 substitute for alcohol when extracting spagyric tinctures by macerations as in the basic herbal process; however, its cost makes it less attractive to other non-alcoholic processes such as that with olive oil. We find that a good application of glycerol is in the making of spagyric magisteries.
spagyric magistery using glycerol
By Magistery here, we mean the combination of purified water soluble salts, steam distilled essential oils and a mercury medium. We start with the separation of essential oil via steam distillation. This can also be done by store bought essential oils provided they are steam distilled and not extracted by chemical solvents.

Rosemary
The plant residues are calcined and the water soluble salts extracted, purified then roasted at 400°C to evaporate the crystallizing water. The oils are added to the warm salts, then glycerol is added. Glycerol substitutes alcohol here 1:1. An example below is rosemary magistery with 5 ml of steam distilled essential oil, 3 grams of water soluble salts, and 100ml of glycerol.

The three essentials of Rosemary combined.
The combination is subjected to a warm circulation at 60°C. In this case, a strong red color develops and more salts get dissolved in the mix than with alcohol.

Circulation under moderate heat.

After 1 day of circulation. Most salts have dissolved.
conclusion
We presented alternatives to alcoholic spagyric products for the purpose of extraction while avoiding the use of ethyl alcohol. This makes basic plant preparations available to a wider range of students of alchemy and general herbalism. We hope further research can reveal other solvents or interesting products and combinations in the work of the plant or the mineral Kingdoms.